The French system for preventing and managing shortages

28 May 2020
BLUEREG GROUP
The French regulation on the prevention and management of stock shortages of medicinal products is part of the European legal framework defined by Directive 2001/83/EC, one article of which (81) establishes the principle of the responsibility of manufacturers and distributors in the appropriate and continuous supply of the market and another (23a) provides for mandatory notification of the national competent authority by the industrial operator of any temporary or permanent unavailability of the medicinal product (no later than two months before the interruption in the marketing of the medicinal product).
The fight against drug shortages has been reinforced by Law n°2016-41 of January 26, 2016 to modernize our health system, known as the "Health" law. This law placed medicines of major therapeutic interest (MITM) at the heart of the system for preventing and managing stock shortages.
____________________
MITM (Article L5111-4 of the "Health" Act) means the drugs or classes of drugs for which an interruption of treatment :
- is likely to jeopardize the vital prognosis of patients in the short or medium term,
- or represents a significant loss of opportunity for patients in terms of the disease's potential for progression].
Including a section of that law that reads as follows: Article L.5121-31
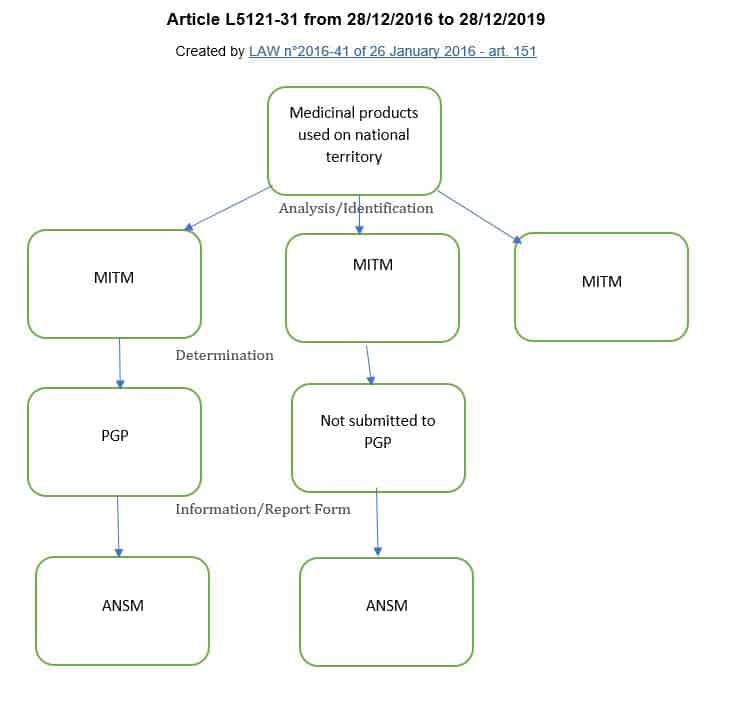
For the medicinal products of major therapeutic interest mentioned in Article L. 5111-4 for which, because of their characteristics, stock shortage or the risk of stock shortage presents a serious and immediate risk for patients, marketing authorisation holders and pharmaceutical companies operating medicinal products draw up and implement shortage management plans whose purpose is, in the interest of patients, to prevent and remedy any stock shortage...
In 2018, the ANSM Agence Nationale de Sécurité du Médicament et des produits de santé : French National Agency for the Health Safety of Medicines and Health Products identified 871 reports of stock shortages and risks of stock shortages of medicines in its annual activity report. This number is an increase of more than 64% compared to the 530 reports recorded in 2017, which were already a record.
Thus, in the context of prevention and management of drug shortages, the draft Social Security Financing Law (PLFSS) for 2020 includes (in its Article 34) several provisions with the aim of strengthening the regulation of stock shortages of essential medicines.
These include the following:
- The introduction of an obligation for laboratories to build up a safety stock for medicines whether they are MITMs or not (which modifies article L. 5121-29 of the Public Health Code (CSP)). It is planned that this obligation will come into force from 30 June 2020.
- The reinforcement of the obligation of laboratories to inform the ANSM in the event of rupture or risk of rupture of a MITM (which modifies article L. 5121-32 of the CSP by introducing marketing authorisation holders as additional actors of information to the ANSM)
-The introduction of an import obligation for the defaulting company. (with a rewriting of article L. 5121-33 of the CSP)
- The tightening of the penalties that may be imposed on manufacturers who do not comply with their obligations regarding the appropriate and continuous supply of the national market (creation of a new Article L. 5423-9 of the CSP entirely devoted to breaches subject to financial penalties).
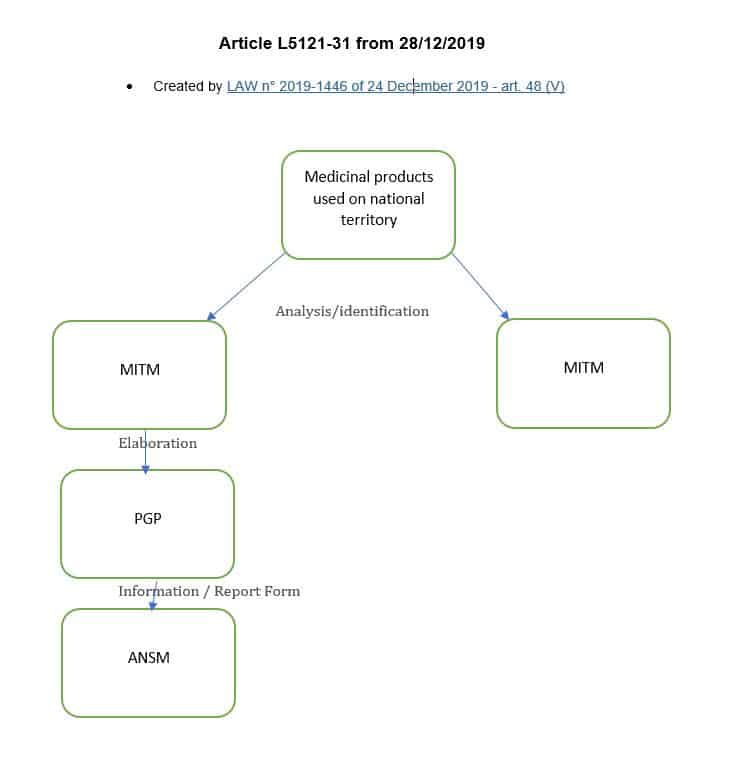
And the modification of the above-mentioned article L.5121-31, by the LAW n° 2019-1446 of December 24, 2019 according to :
For the medicinal products of major therapeutic interest mentioned in Article L. 5111-4 , marketing authorisation holders and pharmaceutical companies operating medicinal products draw up and implement shortage management plans, the purpose of which is, in the interest of patients, to prevent and remedy any stock shortage...
The removal in the section of the phrase "for which, because of their characteristics, the shortage or risk of a shortage presents a serious and immediate risk to patients," makes it mandatory that a shortage management plan (PGP) be developed for any MITMs.
Indeed in the previous version of this article, MITMs were not necessarily subject to a PGP, the manufacturer was responsible for identifying the MITM subject to PGP according to the characteristics of these drugs specified by decree (decree of July 20, 2016) and their belonging to the therapeutic classes of MITMs defined by order of the Minister of Health (order of July 27, 2016).
SCHEMATICS FOR AN INDUSTRIALIST :
As an indication, the drafting of a PGP can be done according to the following plan:
I General information on the MITM
II Fragility elements identified during the risk analysis (from manufacturing to distribution of the MITM)
III Measures in place to prevent and manage stock-outs and limit tensions or even stock shortages
- Monitoring, prevention, actions implemented to mitigate rupture
IV Measures envisaged in the medium term (if current measures are deemed insufficient)
Add the frequency of revision of the PGP, the references and definitions and as an annex the ANSM
References:
- Public Health Code and in particular articles :
Art. L.5111-4
Art. L.5121-31 & Art. L.5121-32
- Law n°2016-41 of January 26, 2016 to modernize our health system - Art. 151 and Law n° 2019-1446 of December 24, 2019 - Art. 48
- Decree n°2016-993 of July 20, 2016 relating to the fight against disruptions in the supply of medicines
- Order of 27 July 2016 setting the list of therapeutic classes containing medicines of major therapeutic interest mentioned in Article L. 5121-31 of the Public Health Code
- LEEM circular: information_types_potential_integrees_to_pgp_pj_reco_pgp_2019
- ANSM annual activity report 2017




