PRIME EU and Breakthrough Therapy Designation US
The PRIORITY MEDICINES scheme, also known as PRIME, is a scheme launched in March 2016 by the EMA to enhance support for the development of medicines that target an unmet medical need with a high public health potential.
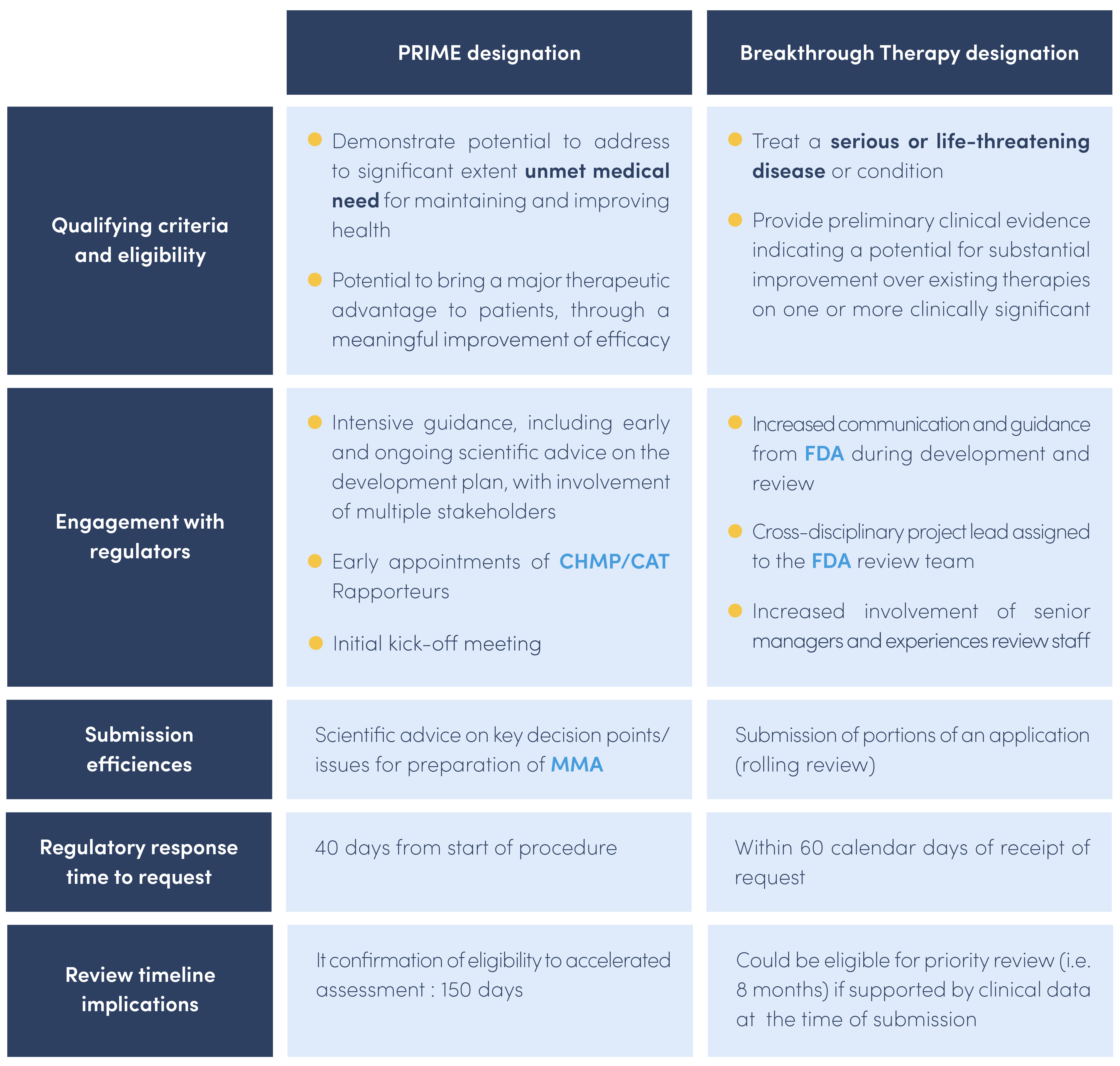
This scheme is voluntary and allows enhanced and early interactions between developers of medicines and the EMA to optimize development plans to generate robust data on the medicine’s benefits and risks. The PRIME designation allows accelerated assessment of medicine applications.
When is the PRIME designation granted ?
The PRIME designation is granted when the two following criteria are met :
- The medicine targets an unmet medical need.
- A justification must be provided to explain what the unmet need is and how it will be addressed with evidence.
Once the PRIME designation has been granted to a candidate medicine, there are several benefits for the sponsor during development :
- A rapporteur from the CAT is appointed by the EMA to provide continuous support and guidance that would allow the sponsor to build a robust data package for a MAA
- The EMA organizes a kick-off meeting with the CAT rapporteur and a multidisciplinary group of experts to provide preliminary guidance on development plan, discuss key development steps and regulatory strategy.
- The EMA assigns a dedicated single contact point who will coordinate regulatory support throughout the scheme
- The EMA provides scientific advice at key development milestones and on key issues.
- Confirmation of accelerated assessment at the time of MAA
The FDA has a similar designation to expedite development and review of drugs that are intended to treat serious conditions and preliminary clinical evidence showing that the medicine may demonstrate substantial improvement compared to currently available therapies. This is known as Breakthrough Therapy Designation. Benefits are the same as PRIME.
PRIME scheme and accelerated assessment
Once the PRIME designation has been granted to a medicine, one of the benefits is to be eligible for accelerated assessment at the time of marketing authorization application. Applicants for accelerated assessment should justify the fact that the medicine is expected to be a major public health interest particularly from the point of view of therapeutic innovation.
Sponsors wishing to apply for an accelerated assessment should send a request to the EMA at least two or three months before submitting their MAA.
Accelerated assessment reduces the time of assessment by the CAT and CHMP from 210 days (standard MAA evaluation) to 150 days.
Once the Breakthrough Therapy designation has been granted to a medicine by the FDA, submission of portions of data before marketing authorization application is possible. This is the rolling review process.
The table below shows the mains differences between the PRIME designation and the Breakthrough Therapy designation.
How can BlueReg help ?
At BlueReg, our team of experts has an excellent knowledge of the EU regulation and an excellent understanding of the scientific and development challenges that developers can face with innovative medicines including ATMPs. Our regulatory experts also have an excellent understanding of EMA expectations for these products and can help medicine developers defining the best suitable regulatory strategy.
We can advise developers on very early and late stage of the development process on several innovative medicines. We work with sponsors on the risk-based approach and we select the appropriate tools at the right time to design the best regulatory strategy and get a successful marketing authorization.
BlueReg has a keen interest in innovative medicines and worked on many innovative medicines so far. Experience gained from these projects is applicable to the issues you can face when developing an ATMP. We can guide you at every regulatory step described above and beyond (discussion on the risk-based approach, pediatric investigation plan, orphan drug designation and post-approval activities).
Learn more about our services for ATMPs
Consultancy companies are very sought by non-European companies who do not have European affiliates. If SME companies are not established in the EU/EEA, SME incentives can be accessed through BlueReg regulatory consultancy who has the SME status. BlueReg and their EMA approved SME clients have access to several benefits such as administrative, regulatory, and financial support.
