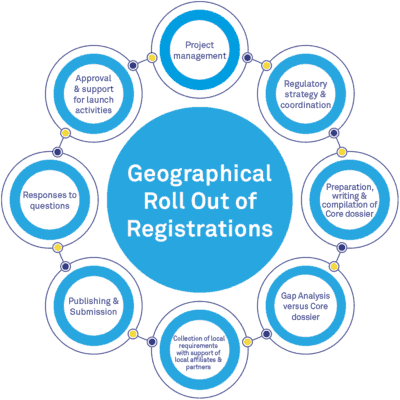
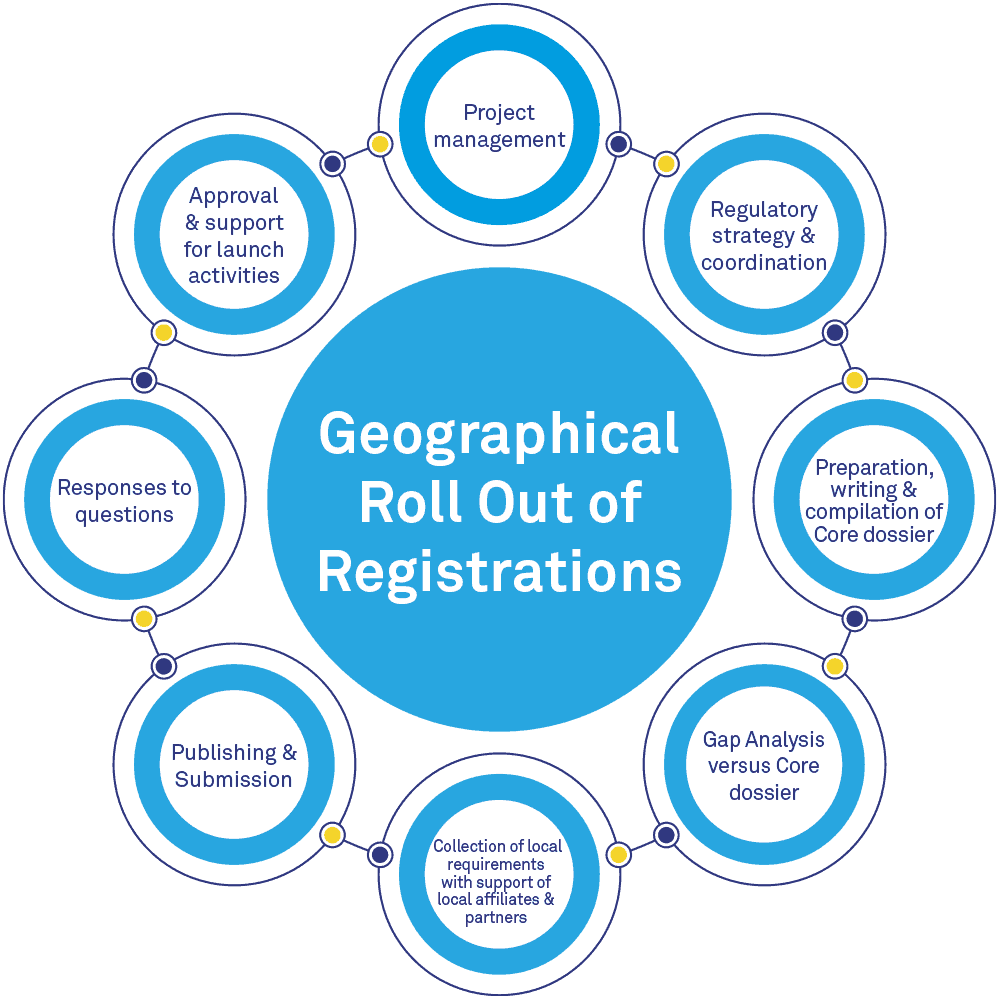
As an outsourcing and consulting services company, BlueReg is your strategic partner to meet your goals for all medicinal product registrations worldwide. BlueReg provides comprehensive regulatory affairs services, in global regulations and intelligence, across all regions of the world, supported by the expertise of our consultants and our international qualified partners.
BlueReg will support your geographic registration roll out in ICH and non-ICH regions by providing you with advice and regulatory support on the various critical steps of your regulatory plans. We provide flexible operational platforms to meet your company’s needs for any pharmaceutical forms of drugs and biologicals.