Pharmaceutical drug launch strategy for promotional materials in EU
25 November 2021
BlueReg
As a pharmaceutical company, you want to make sure that your launch strategy in Europe is as successful as possible. It's important to be aware of the regulatory affairs involved and how they can impact your product launches. In addition, it's essential to have an understanding of what promotional compliance entails so that you don't run into any issues with regulators or healthcare providers.
This blog post will provide a comprehensive overview of the promotional communication associated to your drug launch strategy in Europe and explain what promotional compliance means for those looking to market their products in this region. It will also touch on some challenges faced by companies marketing drugs across different European countries and explain why having a solid plan going into each country is critical for success.
We are going to introduce you to the exciting topic of drug launch with an emphasis on compliance of advertising activities for pharmaceutical products successful launch in Europe such as:
- Giving an insight into why promotional activities have become so key to the success of drug launch and launch strategy
- Evaluate the added value to having a third party on board managing the compliance of your advertising campaigns.
Let’s take a step back and look at some market research that will help us understand why drug launches, and especially marketing activities have become so critical in the overall success of new drugs.
Pharmaceutical industry: A competitive and challenging environment
As you well know, the pharma industry is evolving in an extremely competitive and challenging environment where R&D expenditures often do not yield an acceptable return on investment and there are many reasons for that. We are going to run through some of the major ones.
If we start with the average cost to bring an asset to the market. These costs have been steadily increasing and in fact, market research shows they have almost doubled within the past 7 years moving from 1.3 Bn$ to 2.4 Bn$
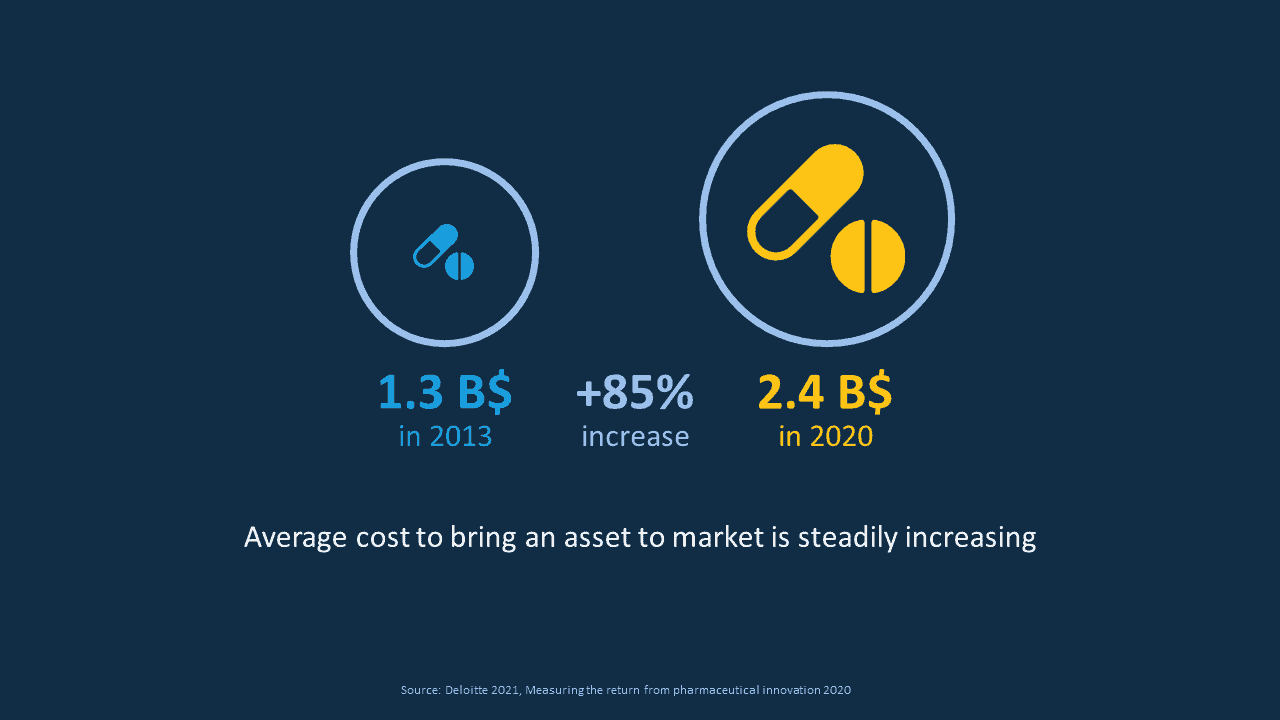
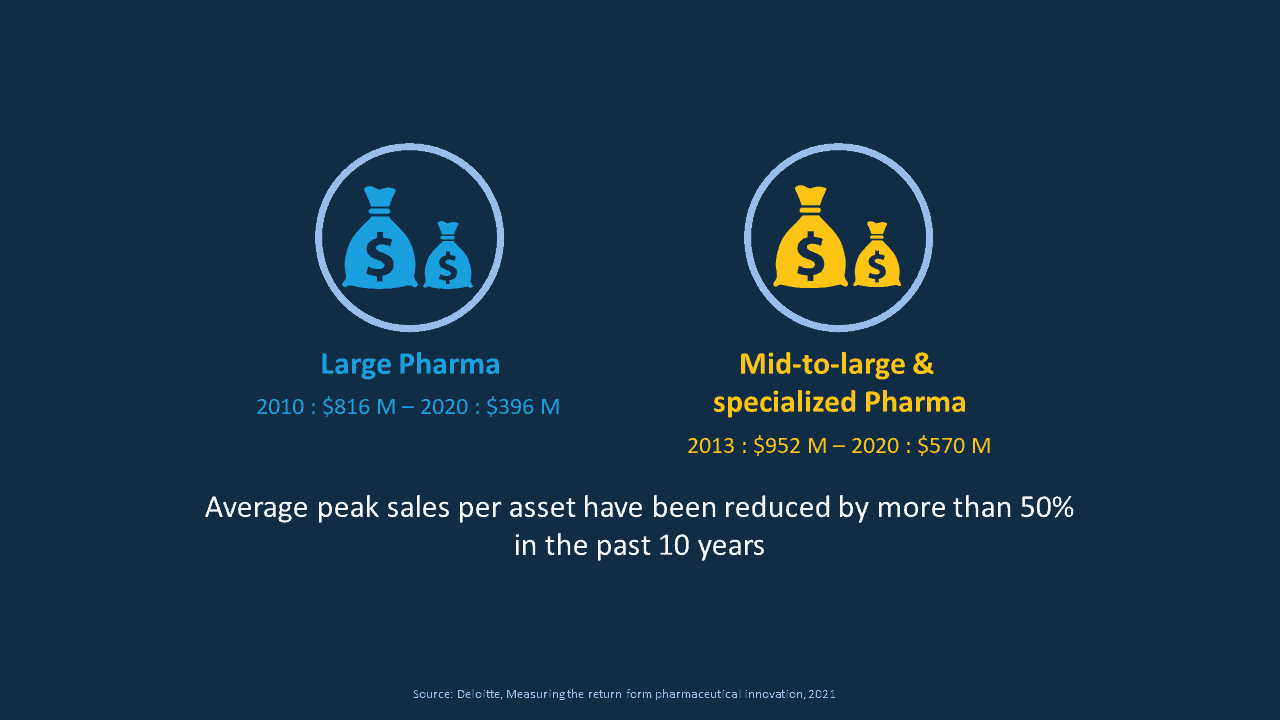
And with that, in contrast, the average peak sales have dropped by more than 50 % in the past 10 years, and what is important to see here is that this figure applies to big pharma but also to mid to large, specialized pharma, meaning companies that focus on a given indication or product.
And to make matters worse…
The average time on which a new drug remains on the market before a competitor arrives has shrunk from 8 down to less than 5 years and peak sales are taking longer to reach.
So of course, there are other reasons such as pressure on price, the role of generics or COVID lately, but taken together, if we consider:
- the rising cost of clinical trials during drug development
- the drop in peak sales due to market realities
- the pressure on time just described
It is pretty easy to understand that, more than ever, a successful launch execution of your product is key to success!
So, what are the figures when it comes to product launches? What real-world data are telling about pharmaceutical companies’ performances?
Drug Launches are more and more difficult to achieve for pharmaceutical companies
I think everybody will agree when we say that successful product launches are more and more difficult to achieve.
- Market Research have shown that nearly half of the drugs between 2009 and 2017 (where we have the whole launch performance picture) have been underperforming as compared to expected sales forecasts.
Of course, there are outliers where performance rates are much better than expected sales, and one typical case is in the field of rare diseases for instance.
But one important point to mention here is that performance in the first year will set the tone for long-term product outlook. In other words, if pharma companies miss expectations in the launch year, they will likely continue to do so in the subsequent years.
This is why it is so important at one point, to invest for success.
Pharma Companies: the need to invest for success
Indeed, product launches are one of the biggest expenses undertaken by pharma companies worldwide and to give you an idea, market research shows that a single product pharma company will spend between 400 and 800 M$ over a 6-year period starting 3 years prior to the launch and most of this money will be dedicated to the product launch success.
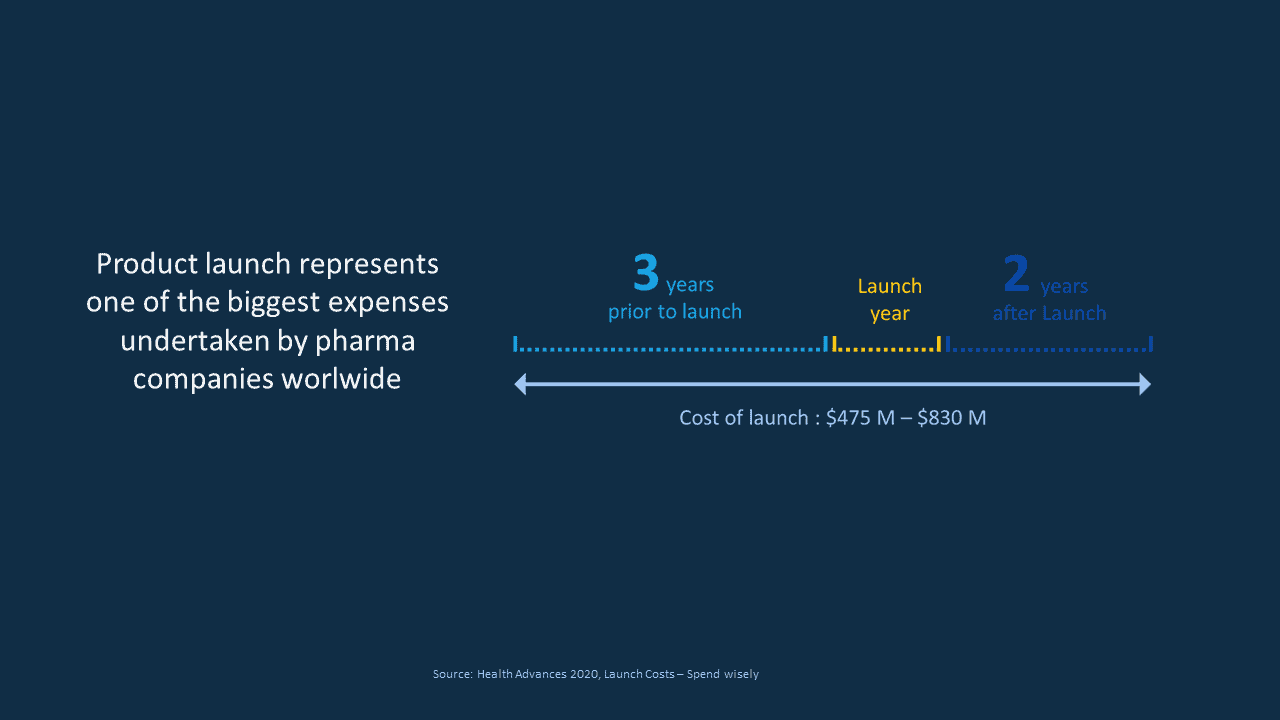
Surely, this is a lot of money but what participates in successful launches in the end?
- Clinical level of efficiency will be key, but nowadays it is not always sufficient. Indeed, there are many examples of successful launches for drugs that were not considered as a major clinical breakthroughs.
- Communication has been a key success factor in these cases but in the end, whatever the situation, effective communication plays a central role in the success of your product launch. And nowadays, for most pharma companies, the importance of targeted, cost effective marketing and outreach campaigns during the pre and the peri launch period has never been greater.
Pharma companies spend billions each year in marketing and some companies even spend more on marketing than R&D to reach commercial success. So yes, communication is key, but you cannot do whatever you want with communication when it comes to medicine.
Medicinal Product Launch: Communication is key but strongly regulated
Indeed, to avoid inaccurate, misleading, or unethical promotion of medicine, promotion is strongly regulated. And to make matters worse, regulation is different from one country to the other.
If we take a quick look at the map representing market share of new drugs launched between 2015 and 2020, it is quite easy to understand that Europe definitely is a market of interest. But it is also an increasingly complex regulatory environment.
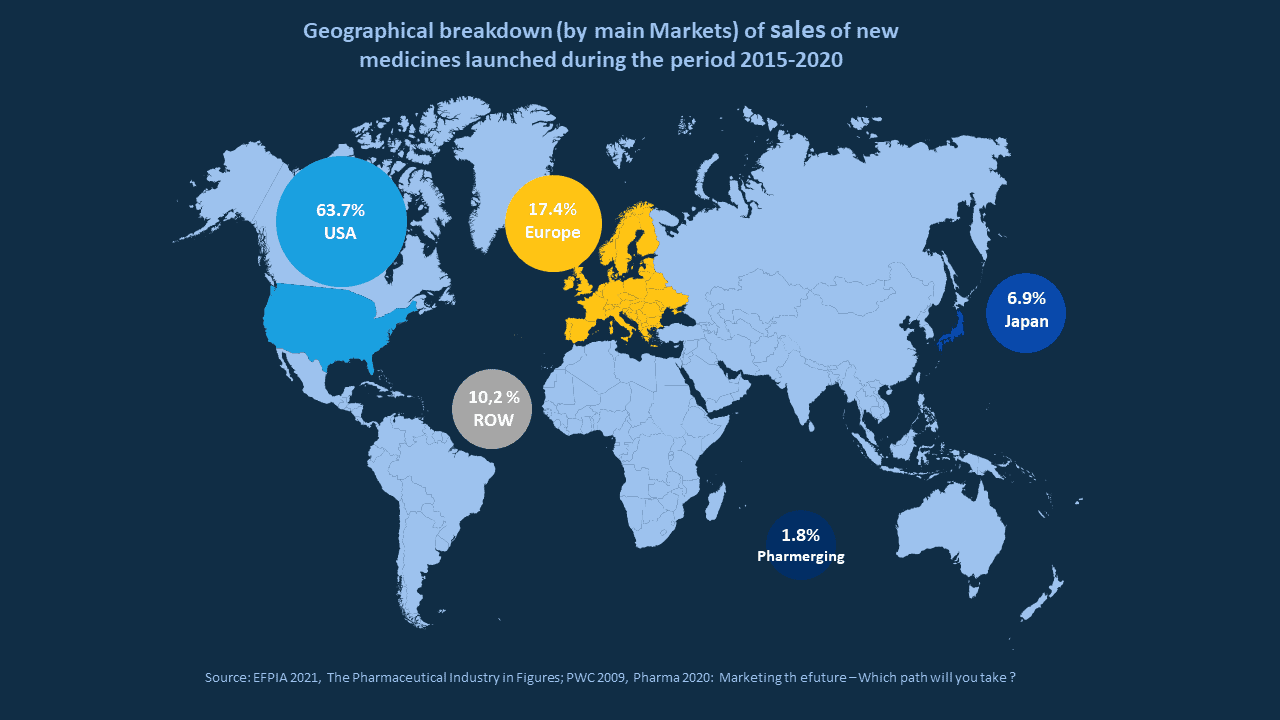
One might think that you can get one pan EU MA through the centralized procedure and that the launch activities, including the marketing activities will be quite straightforward.
But in reality, pharma companies will have to face 30 countries, 30 different local health agencies, the local requirements and their associated deadlines, and all that in as many as 25 different languages. And this also applies to marketing activities and market access.
Promotional Compliance in Europe: Why outsourcing such key activity to facilitate market access?
This brings us now to the last part addressing the question of whether or not, there is an added value to have a third party on board, managing the compliance of your advertising campaigns for successful launch.
Very basically, we have seen that promotional activities can be a competitive advantage and key to the success of pharma companies drug launches. However, we have also seen that launch teams will have to navigate through quite a complex regulatory environment when it comes to marketing in the EU market.
To resume, it is key, and it is complex, so why would you outsource such activity?
Precisely because it is complex and requires rare skills & specific capabilities and your commercial teams may not have at the time of preparing drug launches in EU.
→ Let’s take a concrete example to better understand:
Let’s imagine you are in a mid-size company, with headquarters in the US, preparing for your first product launch in Europe, a launch date well planned, and you are just starting to build your teams in EU.
Depending on the size of your organization, you may be the Global Regulatory Affairs Director or the Regulatory Affairs, Promotion and labelling Director, still based in the US or you are lucky, and your company already have a brand-new affiliate in EU for which you are the Regulatory Affairs Director or the General Manager.
You will have many different objectives to achieve but one of your mission will be to ensure regulatory compliance of marketing activities in the targeted market.
→ How does this translate into responsibilities?
You will be responsible of course:
- for delivering compliant promotional and non-promotional materials
- to deliver them in due time to ensure time to market will be met as per the strategy in all the different target markets as no need to say that delay in marketing campaigns can be a disaster from a business standpoint
- to stay on budget and it can become quite stressful when you think of all the stakeholders you will have to keep an eye on. At the global level you have all the reviewers (regulatory, medical, legal) and marketing stakeholders and at the local level, you will have all these stakeholders in as many countries as you are targeting. Keeping control of the budget under these circumstances can get really tricky
- to contribute to the overall commercial objectives related to the launch of your product in EU.
Taken together, this can be very challenging if you are the only pilot on board. You may, as a small organization, not have the necessary expertise or experience of the EU market or also a lack in resources.
The added value of a third party
This is definitely where it is an added value to have a third party on board helping you in these activities provided that:
1. the third party is specialized in the field with hands on experience
2. the third party is able to provide the required resources in very short notice in all the targeted countries
At BlueReg, we have the necessary expertise and hands-on experiences and the required resources to offer tailored solutions to our clients and we are in fact regularly solicited on these activities: it represents today up to 30% of all the support we are providing.
How do we generally help our clients?
1. One of the key starting point when launching a campaign in EU is to get a clear view on all the global and local regulatory requirements. So, we can provide this kind of analysis which helps our clients identify the gaps and the resources that are missing to be fully operational.
2. We also support our clients in setting up their processes for the review of promo/non promo material at the global and at the local level and this important step is often underestimated by our clients. Though clear process means efficient review!
3. Aside from this, we can also help our clients from a pure operational point of view, for the review itself and the appointment of local responsible person when needed at country level.
Resources is one of the key challenges encountered by our clients when they are preparing for their launch in EU. Indeed, it is very difficult to fulfil all the roles at the global and the local level.
- clients may not have all the affiliates in place
- recruitment may be ongoing or has not even started
- and some roles do not need a full resource initially which makes the recruitment plan very complex.
This is where, again, a third party will really ease up the situation.
What is really appreciated by our client, is the end-to-end solution BlueReg is able to offer:
1. A project Manager can be appointed, and he is the single point of contact coordinating the whole promotional material review in all the targeted countries ensuring delivery of compliant material, on time within budget
2. We then can also allocate a dedicated team of experts in charge of the regulatory/medical review of your promotional material at the global level but also at the local level, in all the targeted markets!
In summary, by collaborating with a consulting company you would benefit from all the resources you may need in almost a blink of an eye.
As a conclusion, we have seen that while preparing for your marketing activities in Europe, it can really be helpful to collaborate with an expert in the field, and we, at BlueReg, offer a one stop shop managing the entire process at global and local level to save your time, secure your deadlines & your budget, ensure peace of mind and compliance for the success of your advertising activities.
You are interested to know more about how BlueReg can support with your launch activities or promotional material compliance ?
Don't hesitate to contact us at contact@blue-reg.com or
References:
· Deloitte 2021, Measuring the return from pharmaceutical innovation 2020
· Infinity Research 2019, Attaining New Product Launch Success in the Pharma Industry.
· Deloitte 2020, Key factors to improve drug launches, 2020.
· Bain & Company 2017, How to make your drug launch a success.
· Health Advances 2020, Launch Costs – Spend wisely.
· McKinsey & Company, First time Launchers in the pharmaceutical company, 2021.
· Pharma Intelligence Informa, Pharma Marketing 2020: maximizing product launch success amid complex legislative change.
· EFPIA 2021, The Pharmaceutical Industry in Figures.
· PWC 2009, Pharma 2020: Marketing the future – Which path will you take?




